Improving Birth Outcomes with Multiple Micronutrient Supplementation
Reported by:
Saima Ahmed
Presented by:
Multiple Micronutrient Supplementation in Pregnancy Technical Advisory Group
The New York Academy of Sciences
Overview
Adequate intake of essential vitamins and minerals is critical for a healthy pregnancy. Unfortunately, many women in low- and middle-income countries (LMICs) struggle to meet the increased dietary demands for a healthy pregnancy through diet alone. Inadequate nutritional intake frequently leads to poor maternal health and adverse birth outcomes, such as maternal mortality; preeclampsia; insufficient gestational weight gain; stunting; low birth weight (LBW); small for gestational age (SGA); and neonatal mortality. Currently, the World Health Organization (WHO) recommends iron-folic acid supplements (IFA) as the routine standard of care in antenatal care programs. However, strong evidence is now available demonstrating the superiority of multiple micronutrient supplements (MMS) over IFA. To help countries determine if they should transition from IFA to MMS in antenatal care, the New York Academy of Sciences assembled a task force. Charged with taking a closer look at MMS, the task force considered several factors, including benefits, risks, and cost-effectiveness. On June 25, 2019, the task force’s findings were presented at the launch of the Special Issue, published in the Annals of the New York Academy of Sciences.
Highlights
- Data from the 2019 Cochrane Review and the 2017 individual patient data (IPD) meta-analysis demonstrate that MMS has significant added benefits to birth outcomes compared with IFA.
- The task force concluded that countries where nutritional deficiencies are prevalent should consider MMS, as it is a cost-effective and safe alternative to IFA.
- The MMS technical advisory group is translating this evidence into practice by assisting in the rollout of MMS demonstration projects in several countries.
- When compared to IFA, routine MMS supplementation does not increase the risk of adverse effects.
- During pregnancy, the risk of exceeding the UL with a micronutrient-rich diet and daily micronutrient supplementation is very low.
Speakers
Robert E. Black, MD, MPH
Johns Hopkins University
Megan Bourassa, PhD
The New York Academy of Sciences
Gilles Bergeron, PhD
The New York Academy of Sciences
Emily R. Smith, ScD, MPH
The Bill & Melinda Gates Foundation and Harvard T.H. Chan School of Public Health
Alison Gernand, PhD
Penn State University
Reina Engle-Stone, PhD
University of California, Davis
Sponsors

For Policy Makers and Program Implementers
Speakers
Robert E. Black, MD, MPH
Johns Hopkins University
Gilles Bergeron, PhD
The New York Academy of Sciences
Megan Bourassa, PhD
The New York Academy of Sciences
Micronutrient Status and the Benefits of MMS on Birth Outcomes
Robert Black discussed the benefits of MMS on birth outcomes. While the 2016 WHO antenatal care guidelines recommend IFA for routine use, the guidelines also state that countries “might consider the benefits of MMS on maternal health to outweigh the disadvantages and may choose to give MMS that include iron and folic acid.” New evidence on MMS has since emerged, and after a thorough review, the Academy’s task force found strong research in support of prenatal MMS. The data showed a high prevalence of multiple micronutrient deficiencies in women of reproductive age (WRA) and pregnant women in LMICs, suggesting that these women could significantly benefit from MMS during pregnancy. A Cochrane review (updated in 2019) demonstrated that MMS was superior to IFA in reducing important adverse birth outcomes, including small for gestational age (SGA) and low birth weight (LBW).
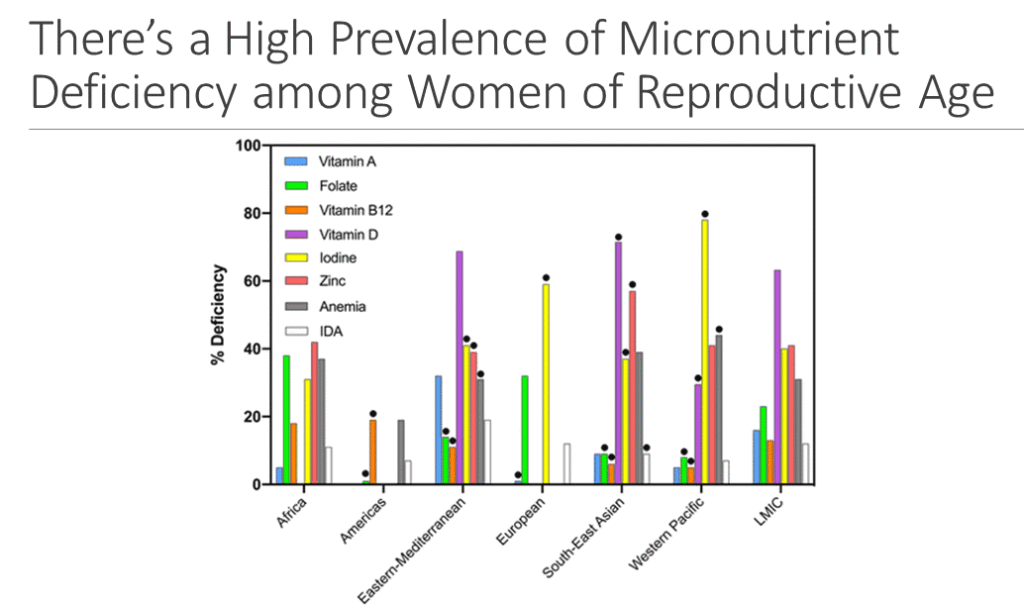
An IPD meta-analysis of several MMS trials conducted in pregnant women provides additional evidence in support of prenatal MMS. Published after the WHO antenatal care guidelines, the analysis showed that women receiving MMS, compared with those receiving IFA, had a significant reduction of SGA and LBW births, very low birth weight (VLBW) births, preterm births, and very preterm births. It also identified a number of subgroups that benefitted from MMS. Additionally, women who were underweight at the onset of pregnancy had a greater reduction in preterm births. Given that complications from preterm births are the leading cause of death in children under five years of age in LMICs, Black stressed the significance of these findings that highlight the potential benefits of MMS and the substantive effects that it can have on birth outcomes. Ultimately, Black concluded that the data from both systematic reviews suggest that countries with high rates of nutritional deficiencies should consider the switch from prenatal IFA to MMS.
Task Force Conclusions and Guidance on MMS in Pregnancy
Megan Bourassa explained that during their review of the evidence, the task force took a closer look at the prevalence of micronutrient deficiencies, cost-effectiveness, and the safety of MMS. They concluded that populations with a high prevalence of nutritional deficiencies might have a greater benefit from MMS. The task force also found that MMS is highly cost-effective in comparison to other antenatal care interventions, such as micronutrient fortification or balanced protein energy supplementation for pregnant women. And after examining the safety considerations, they found no serious side effects associated with the use of MMS. Thus, they concluded that MMS is a cost-effective and safe alternative to IFA, and should especially be considered by countries where nutritional deficiencies are prevalent.
The task force outlined a few questions for countries to consider when making their decision on whether or not to switch from IFA to MMS. The first question is whether the country has a high prevalence of nutritional deficiencies, said Bourassa. Since the WHO did not explain how to define a nutrient deficiency, the task force suggested a list of indicators that might be useful to consider, such as dietary intake, underweight prevalence, and biomarker data, among others. Countries can use these indicators to compile and assess available data to decide whether there is sufficient evidence to make the switch.
To successfully transition from IFA to MMS, countries should consider several factors during the planning process. First, MMS should be built into the existing antenatal care program rather than creating a standalone intervention. Second, policymakers should consider taking the opportunity to assess and strengthen their respective antenatal care programs, including the coverage and adherence to supplementation. If the current program has inadequate coverage, MMS likely will not reach the target population, thus yielding insubstantial results. Countries may also want to consider taking on a small demonstration project to test this in a smaller region to identify any potential issues with the supply and distribution chain. Lastly, as with all public health interventions, it is essential to develop a monitoring and evaluation system to ensure the continued coverage and success of a prenatal MMS intervention.
The Future Direction of the Task Force
Since the release of the Special Issue, the task force has made strides in translating evidence into policy and practice in real world-settings, said Gilles Bergeron. For example, a Technical Advisory Group (TAG) on MMS was formed this year to spearhead the development of a series of technical reference materials. The materials are designed to provide countries with more information on MMS and assist interested countries with the transition from IFA to MMS, and much more. Currently, the TAG is in the process of using the Child Health and Nutrition Research Initiative (CHNRI) methodology to inform the direction of research and investments needed to support the implementation of MMS interventions for pregnant women in LMICs. Bergeron also discussed future directions of the TAG, specifically its partnership with UNICEF and multiple stakeholders, to promote the rollout of MMS through demonstration activities in four countries—Bangladesh, Madagascar, Tanzania, and Burkina Faso—as well as in other potential countries considering the switch.
For Research Scientists and Clinicians
Speakers
Emily R. Smith, ScD, MPH
The Bill & Melinda Gates Foundation and Harvard T.H. Chan School of Public Health
Reina Engle-Stone, PhD
University of California, Davis
Alison Gernand, PhD
Penn State University
Clinical Trials, MMS Adherence, and Adverse Birth Outcomes
The second session focused primarily on information for researchers and clinicians. Emily Smith took a closer look at the results of the clinical trials and discussed the available evidence on side effects and adherence. Her presentation aimed to answer a key question: Is MMS is better than IFA alone for ensuring a positive pregnancy experience? Smith shared results from the most recent and comprehensive reviews on MMS, specifically the 2019 Cochrane Review and the 2017 IPD meta-analysis. The recently updated Cochrane Review evaluated the effects of MMS compared with IFA on pregnancy outcomes, using a total of 20 clinical trials with data from over 140,000 women. Findings showed that MMS resulted in a 12% reduction in LBW and an 8% reduction in SGA births, compared with IFA. While the Cochrane review focused on the overall effects of all available trials, the IPD meta-analysis was primarily aimed at conducting subgroup analyses. This meta-analysis included data from 17 randomized controlled trials from over 100,000 pregnancies in LMICs and found that MMS not only reduces the risk of SGA and LBW, but also reduces the risk of stillbirth, very LBW, early preterm birth, and preterm birth when compared with IFA. The findings from the IPD meta-analysis—26 subgroup analyses were conducted to identify individual characteristics that may further modify the effect of MMS—also showed specific subgroups have a greater benefit from MMS. When compared with IFA, the effects of MMS resulted in a more significant benefit for undernourished women, specifically those who were anemic or underweight (BMI <18.5 kg/m2) or women who gave birth to female infants.
The WHO antenatal care guidelines raised an important point of concern regarding the potential risk of increased neonatal mortality. This concern arose from a subgroup analysis comparing those receiving MMS with 30mg of iron to those in the control group receiving IFA with 60mg of iron. When reviewing the analysis, it was apparent that a few errors and omissions were made. A recent reanalysis of these data, performed by Sudfeld and Smith, included all eligible studies and corrected point estimates and found no increased risk of neonatal mortality associated with MMS. Though limited evidence was available, six of the seven clinical trials (which reported on this outcome) showed no significant differences in the side effects between IFA and MMS. Similarly, differences in adherence rates between IFA and MMS were minimal, with no more than a 2% difference between the intervention groups in 10 trials that reported on adherence. Smith concluded that routine MMS supplementation does not increase the risk of adverse effects, and has a number of additional benefits for mortality and birth outcomes compared with IFA, especially in areas where nutritional deficiencies exist.
The Upper Level: Antenatal Supplements and the Risk of Excess Micronutrient Intake
While understanding the global prevalence of micronutrient deficiencies in LMICs and the substantial benefit MMS can provide to alleviate the burden, there can be health risks when intake regularly exceeds a high amount of nutrients, otherwise known as the upper intake level (UL). Alison Gernand outlined what is known about these risks in pregnancy. The WHO defines UL as the “maximum intake from food, water, and supplements that is unlikely to pose the risk of adverse health effects”. It is important to note that the UL values are set for healthy people with good baseline micronutrient status, not for those with deficiencies or medical conditions. Since the prevalence of deficiencies is high in LMICs countries, an intake higher than the UL may be warranted for a limited timeframe to correct the deficits. Little is known about pregnancy-specific risks, so the ULs are the same for pregnant and non-pregnant women, except vitamin A, due to the possibility of birth defects. In general, there is little to no risk of excessive intake for several vitamins—including thiamin, riboflavin, vitamin B12, and vitamin C—from large supplemental doses. Potential adverse effects from excess micronutrients such as niacin, folate, and iron are only due to supplement intake.
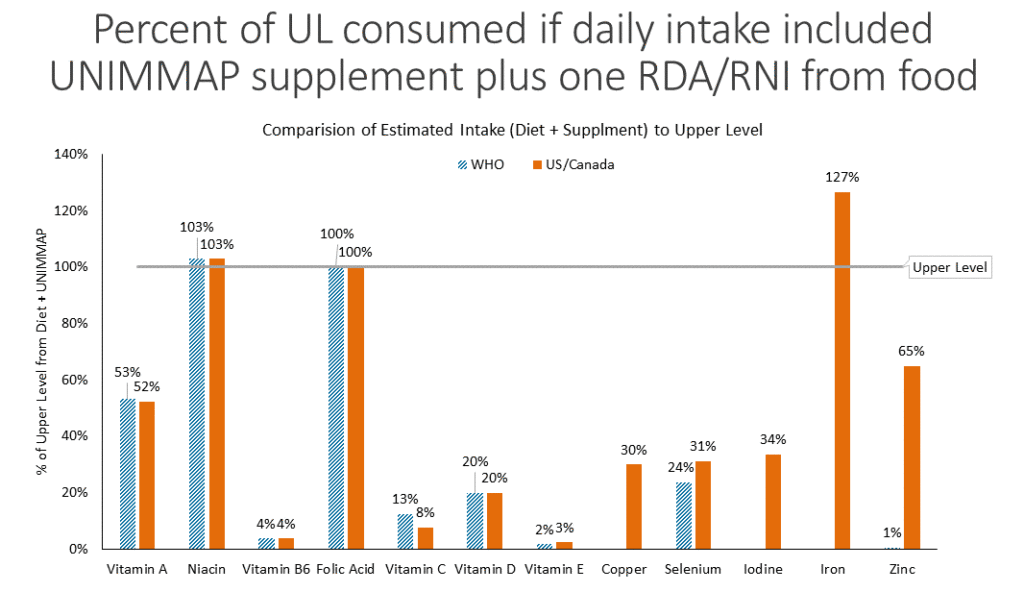
To assess the risk of reaching the UL with an adequate diet, Gernand compared the Reference Daily Intake (RNI) or Recommended Dietary Allowance (RDA), and compared the amount in the UNIMMAP supplement to both the Institute of Medicine (IOM) and WHO UL values. The results showed that folate intake reached the UL, while iron and niacin slightly exceeded it, with known risks of each nutrient to be mild. For folate, an excess can mask vitamin B12 deficiency; otherwise, toxicity due to excess intake has not been known. Risks due to excess iron intake, specifically nausea and vomiting, can be eliminated if the supplement is taken with food. Finally, for niacin, the risk of flushing resulting in skin reddening and itchiness is due to nicotinic acid, found only in supplements, not in food. Gernand said that limited information on pregnancy-specific risk from excess intake is available, stressing the urgency for more published data. Overall, the risks of exceeding the UL during pregnancy from a micronutrient rich diet and daily MMS are very low and should not result in adverse effects.
Cost Analyses: Replacing IFA with MMA During Pregnancy
According to new evidence compiled by the task force, MMS has additional benefits over IFA, but the tablets are more costly. While there is sufficient evidence to support the transition of IFA to MMS, policymakers need to consider not just the benefits, but also the associated costs. In her presentation, Reina Engle-Stone asked if MMS is a worthwhile investment. With her team at UC Davis, Engle-Stone developed a model to estimate the effects, cost, and cost-effectiveness of replacing IFA with MMS within the context of a supplementation distribution program in Bangladesh and Burkina Faso. A hypothetical one-year scenario with 100% coverage was also applied to both countries using their current national levels of IFA coverage, assuming complete adherence to the recommended regimen (i.e., consumption of 180 supplements per pregnancy). The model used baseline demographic characteristics from the Lives Saved Tool (LiST) and effect sizes from the IPD meta-analysis to generate the marginal effects of replacing IFA with MMS on mortality, adverse birth outcomes, and disability-adjusted life years (DALYs) averted, in both rural and urban settings.
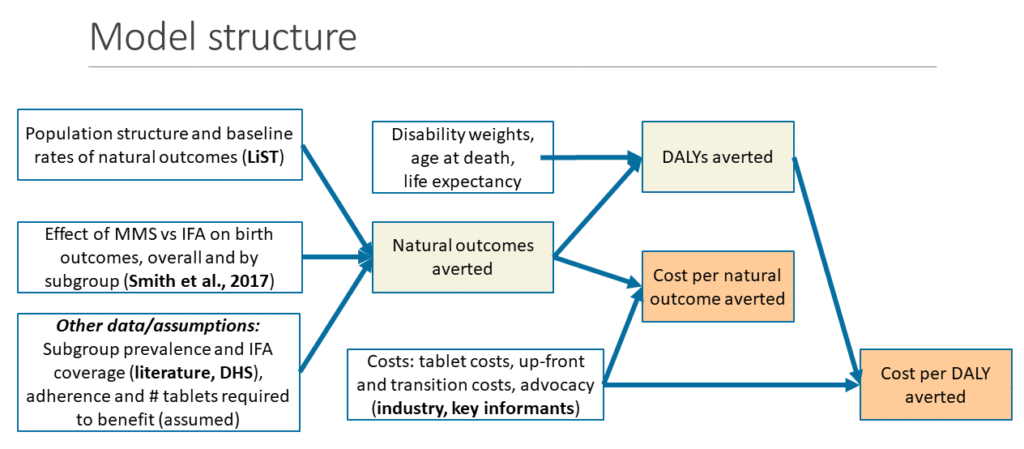
The results showed replacing IFA with MMS could avert over 15,000 deaths and 30,000 cases of preterm birth annually in Bangladesh, and over 5,000 deaths and 5,000 cases of preterm birth in Burkina Faso, assuming 100% coverage and adherence. The cost per death averted was estimated to be $175-$185 in Bangladesh and $112-$125 in Burkina Faso. Lastly, the cost per DALY averted ranged from $3-$15, depending on the country and consideration of sub-groups. Engle-Stone noted that the estimate is very sensitive to the cost of the tablet. For one, the costs associated with shifting from IFA to MMS will be significant, given that MMS are approximately 35% more costly than IFA tablets. Based on the hypothetical scenario, a complete switch to MMS in Bangladesh given current coverage levels (50% nationally) would cost approximately $1.7 million. In a scenario assuming 100% coverage, where all women receive and consume 180 tablets, the additional cost to replace the IFA with MMS would increase to $2.7 million. A complete switch in Burkina Faso with current coverage levels (10.2% nationally) would cost approximately $60,000 and would rise to $600,000 for 100% coverage.
In sum, the switch would come at an added cost, and if the cost of the supplement rises, so will the cost-effectiveness. However, an increase in demand of MMS with improvements in program delivery and supplement adherence could improve the cost-effectiveness. Engle-Stone noted that further research is needed to provide a more realistic scenario for the transition from IFA to MMS, specifically on the delivery platform performance and supplement adherence. Nonetheless, the cost-effectiveness of this short, one-year analysis suggests that policymakers should consider adopting the underlying model with necessary modifications to fit their context and use it to better inform policy discussions around the shift from IFA to MMS.