Resolution of Inflammation, Infection and Tissue Regeneration
Reported by:
Alan Dove
Presented by:
The New York Academy of Sciences
Overview
For centuries, physicians and scientists have thought of inflammation as the body’s acute response to infection or injury, but in recent decades it’s become clear that chronic inflammation drives pathologies as diverse as cancer, diabetes, and Alzheimer’s disease. Controlling this aberrant inflammation, however, has proven difficult. Conventional anti-inflammatory drugs work by antagonizing the body’s pro-inflammatory hormones, but that approach also suppresses immunity, opening the patient to secondary infections. A newer strategy relies on recently discovered resolution mediators, compounds that the body makes naturally to resolve inflammatory responses without suppressing other parts of the immune system. Drugs targeting this process have shown immense potential to treat many of the world’s most serious diseases, with fewer side effects than existing therapies.
On June 25–26, 2018, the New York Academy of Sciences hosted Resolution of Inflammation, Infection and Tissue Regeneration, a symposium featuring many of the top researchers in the rapidly developing field of resolution pharmacology. In two days of oral presentations, a poster session, and an extensive panel discussion, speakers and attendees reviewed the biggest advances and challenges in resolution biology. The meeting covered the basic biology of inflammation and its resolution, studies on animal models of chronic and acute diseases, and clinical trials of promising new inflammation-resolving drugs.
Speakers
Nan Chiang, PhD
Brigham and Women’s Hospital, Harvard Medical School
Michael Conte, MD
University of California, San Francisco
Jesmond Dalli, PhD
William Harvey Research Institute, QMUL
Gabrielle Fredman, PhD
Albany Medical Center
Catherine Godson, PhD
University College Dublin
Dipak Panigrahy, MD
Beth Israel Deaconess Medical Center, Harvard Medical Center
Paul Ridker, MD, MPH
Brigham and Women’s Hospital, Harvard Medical School
Charles Serhan, PhD, DSc
Brigham and Women’s Hospital, Harvard Medical School
Patricia Sime, MD
University of Rochester School of Medicine
Matthew Spite, PhD
Brigham and Women’s Hospital, Harvard Medical School
Ira Tabas, MD, PhD
Columbia University
Mark Tepper, PhD
Corbus Pharmaceuticals
Kevin Tracey, MD
The Feinstein Institute for Medical Research
Thomas Van Dyke, DDS, PhD
Forsyth Center for Clinical and Translational Research
Sponsors
eBriefing Support
Bronze Sponsor
Academy Friends
Additional Support Provided by
Resolution Mediators and Mechanisms in Inflammation: Leads for 21st Century
Speakers
Charles Serhan
Brigham and Women’s Hospital, Harvard Medical School
Charles Serhan, from the Brigham and Women’s Hospital and Harvard Medical School, opened the meeting with a keynote presentation that spanned the history of resolution physiology, a field he pioneered. Physicians and scientists have known about inflammation since antiquity, primarily as an acute condition associated with injury and infection. In recent years, however, biologists have come to understand that chronic inflammation underlies many non-communicable diseases, including cancer, diabetes, Alzheimer’s disease, and Parkinson’s disease.
Serhan has focused on how acute inflammation normally resolves, and how this process sometimes malfunctions, leading to chronic inflammation. When he entered the field, “in the textbooks, the resolution of acute inflammation was thought to be a passive event,” Serhan said. Pro-inflammatory molecules became diluted over time, bringing the inflammation to an end. Through an extensive series of cell culture, animal, and human studies, Serhan and his colleagues have overturned that model, showing that multiple families of pro-resolving molecules actively antagonize the inflammatory process and promote healing. These small fatty acid-derived molecules, now known as lipoxins, resolvins, protectins, and maresins, act through specific cellular receptors to orchestrate a complex switch from inflammation to resolution.
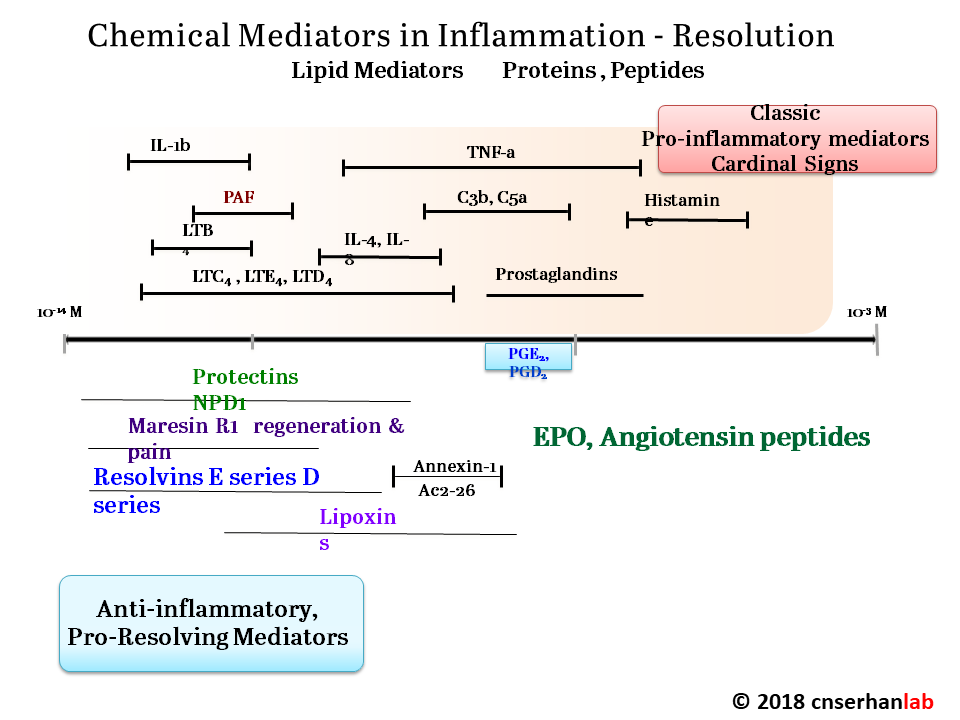
Serhan, like many others at the meeting, is now exploiting those findings to design new drugs that could treat a wide range of chronic conditions far more effectively than current anti-inflammatory compounds, with fewer side effects. “This is really a paradigm shift in thinking about how to treat inflammation, using agonists to stimulate resolution rather than inhibitors that eventually become immunosuppressive,” said Serhan.
Speaker Presentation
Further Readings
Serhan
Serhan, C.N., and Levy, B.D.
Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators.
J. Clin. Invest. 2018;128(7):2657-2669.
Serhan, K., Gartung, A., and Panigrahy, D.
Prostaglandins Other Lipid Mediat. 2018;137: 40-45.
Zhang, L., Terrando, N., Xu, Z.-Z., et al.
Front Pharmacol. 2018; 9, 412.
Session 1: The Fire Inside
Speakers
Dipak Panigrahy, MD
Beth Israel Deaconess Medical Center, Harvard Medical Center
Ira Tabas, MD, PhD
Columbia University
Cancer Progression: Failure to Resolve?
Dipak Panigrahy, of Beth Israel Deaconess Medical Center and Harvard Medical School, started the meeting’s first session by discussing his work on inflammation resolution in cancer. Researchers first discovered the link between inflammation and tumor growth in the late 19th century, but only recently has it become clear that inflammation is an essential initiator of at least some types of cancer. A mouse model of pancreatic cancer, for example, does not get the disease unless it first develops pancreatitis.
Unfortunately, most current anti-inflammatory drugs suppress the immune system, allowing established tumors to become even more aggressive. To find better solutions, Panigrahy began dissecting the mechanisms linking cancer and inflammation. He discovered that cancer-killing interventions such as chemotherapy leave behind debris from the killed tumor cells. In a mouse model of ovarian cancer, that debris worsens the disease. “So the debris will stimulate the tumor growth. It’s kind of a double edged sword of cancer therapy,” said Panigrahy. Adding specialized pro-resolving mediator molecules (SPMs) stimulates phagocytic cells to engulf the cellular debris, inhibiting further tumor growth. Treatments that boost SPMs could work synergistically with chemotherapy to attack numerous types of cancer.
The Interplay between Efferocytosis and Inflammation Resolution
Ira Tabas from Columbia University continued the theme of clearing cellular debris, with a presentation about his group’s work on the process. Macrophage cells function as the body’s garbage collectors, engulfing the remains of dead cells through efferocytosis. The process is critical for healing damaged tissue and resolving inflammation, but it requires intensive metabolic management by the macrophage. “When a macrophage is eating multiple dead cells … it might be like you sitting down eating 20 filet mignons, and then half an hour later eating 20 more,” said Tabas, adding that “it’s a tremendous metabolic load.”
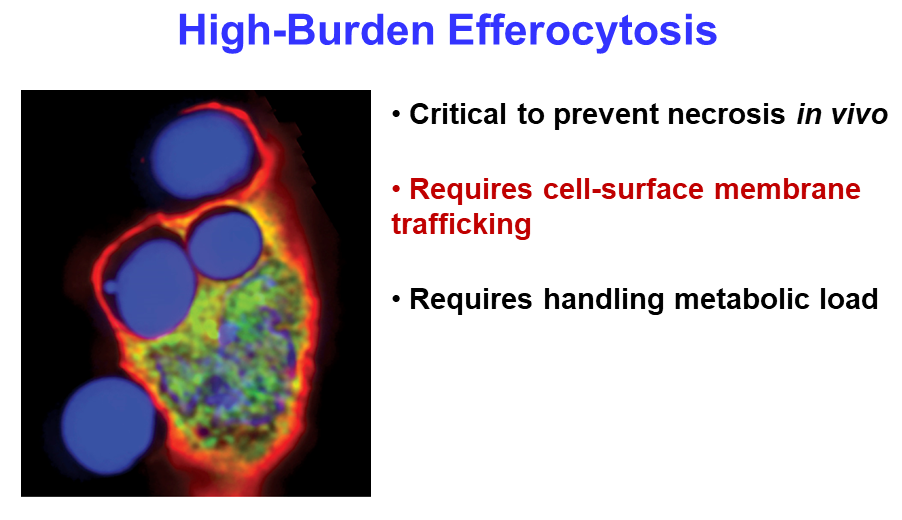
Tabas’s team studies efferocytosis in mouse models of heart disease. They found that eliminating a single gene in the animals blocks efferocytosis and leads to much worse disease, highlighting the importance of efferocytosis in resolving inflammation. Next, the researchers used an elegant in vitro assay to demonstrate that the degradation products from engulfing one dead cell regulate the macrophage’s ability to engulf a second one. The macrophages use another tightly regulated process to recycle the membranes of the dead cells. Together, the mechanisms explain how macrophages can keep their efferocytosis rates as high as possible without becoming metabolically overloaded.
Speaker Presentations
Further Readings
Panigrahy
Chang, J., Bhasin, S.S., Bielenberg, D.R., et al.
Chemotherapy-generated cell debris stimulates colon carcinoma tumor growth via osteopontin.
FASEB J. 2018: fj201800019RR.
Serhan, K., Gartung, A., and Panigrahy, D.
Prostaglandins Other Lipid Mediat. 2018;137:40-45.
Yang, H., Wang, W., Romano, K.A., et al. (2018).
Sci Transl Med. 2018; 10(443).
Tabas
Ghorpade, D.S., Ozcan, L., Zheng, Z., et al.
Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance.
Nature. 2018;555(7698):673–677.
Ozcan, L., Ghorpade, D.S., Zheng, Z., et al.
Cell Rep. 2016;15(10): 2214–2225.
Subramanian, M., Ozcan, L., Ghorpade, D.S., et al.
PLoS ONE. 2015;10(8): e0135842.
Session 2a: Vessels in Distress
Speakers
Michael Conte, MD
University of California, San Francisco
Gabrielle Fredman, PhD
Albany Medical Center
Paul Ridker, MD, MPH
Brigham and Women’s Hospital, Harvard Medical School
Providing Proof of Principle for Atherosclerosis, Lung Cancer, Kidney Disease, and Osteoarthritis: Lessons from CANTOS
Paul Ridker from Brigham and Women’s Hospital began the second session with a keynote address on the CANTOS clinical trial. This trial sought to address several questions about inflammation resolution in cardiovascular disease.
Atherosclerosis is a leading cause of vascular disease worldwide. Researchers have identified numerous factors that predict the onset of atherosclerosis, including high blood pressure, cholesterol, and markers of chronic inflammation, but it remains unclear which of these factors are causative and which merely correlate with pathogenesis.
Current treatments for atherosclerosis focus on lowering blood pressure and cholesterol levels, but Ridker and his colleagues wanted to target inflammation instead. To do that, they used a human monoclonal antibody drug called canakinumab, which binds the pro-inflammatory protein interleukin-1beta. IL-1beta triggers a series of signals that promote inflammation and also increase blood levels of C-reactive protein (CRP), a biomarker of atherosclerosis. A pilot study on 1,400 patients sought to determine how much canakinumab it would take to reduce patients’ CRP levels to baseline, but the drug was so powerful that it worked at all doses tested.
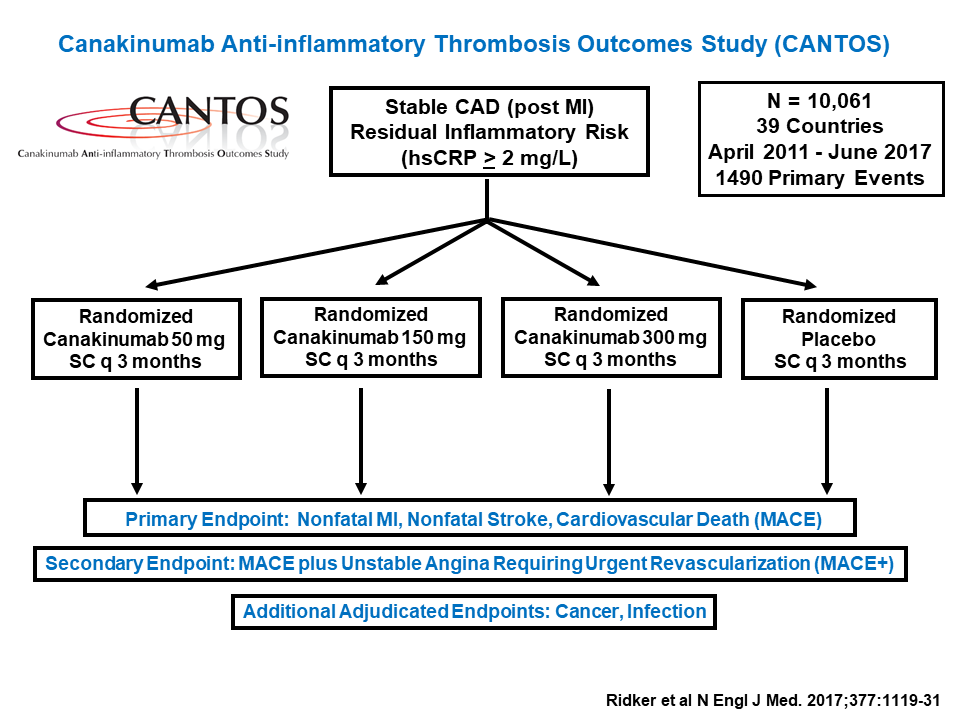
Based on those data, Ridker’s team designed a much larger trial, enrolling over 10,000 patients. The participants all had high CRP levels even after aggressive cholesterol-lowering therapy, indicating that they were still suffering from chronic inflammation. Those treated with canakinumab showed dramatic reductions in CRP, and concomitantly lower rates of cardiovascular events over the trial’s seven-year run, compared to controls who received a placebo. Crucially, treated patients showed no signs of immunosuppression, and most of the drug’s side effects were mild or even beneficial. For example, patients in the treatment groups had significantly lower rates of overall cancer mortality and chronic kidney disease than controls.
The results show that atherosclerosis treatment plans should focus on both cholesterol and inflammation. “If we’re going to beat this disease, we have to [target] both of these processes,” said Ridker.
Resolution of Vascular Injury: Mechanisms and Therapeutic Implications
Michael Conte, of the University of California, San Francisco, addressed what happens in the next step of modern vascular disease management: surgery. “It really doesn’t matter what we do when we touch blood vessels, whether we inflate a balloon, scrape out plaque, do a bypass graft or insert a catheter, we are faced with a scarring response … that has a classic inflammatory and resolution phase,” Conte explained.
In animal models of vascular injury, damaged blood vessels become inflamed, then produce specialized pro-resolving mediators (SPMs) to resolve the inflammation. Conte’s group also found that delivering extra SPMs locally to the site of injury significantly improves inflammation resolution and healing.
To put that finding into practice, the investigators have developed thin film polymers that can release SPMs directly into a vessel over time. They wrap the film around the blood vessel during surgery and leave it in place to deliver the molecules for days afterward. The system reduces graft thickness in a rabbit bypass graft model, and improves outcomes in mouse models of aneurysm surgery and thrombosis. Conte is also conducting a clinical trial testing the safety of naturally isolated SPM-stimulating compounds in humans.
Dysregulation of Resolution Pathways in Atherosclerosis
Gabrielle Fredman from Albany Medical Center continued the vascular theme, discussing her work on how dead cells get removed from atherosclerotic plaques. As a plaque develops on the wall of an artery, the cells at the center begin to die. This weakens the structure of the plaque and makes it more likely to rupture. Fredman studies this process in genetically modified mice that develop atherosclerosis when fed a high-fat diet. Treating these mice with SPMs decreases necrosis in their plaques, but how?
When cells die through apoptosis, or programmed suicide, macrophages rapidly engulf the resulting debris. Fredman and her colleagues found that cells undergoing necroptosis, such as those in the core of a plaque, are apparently much less appetizing. Macrophages fed necroptotic cells take them up much more slowly and in smaller pieces. “This resembles something like a nibbling process rather than a whole engulfment process,” said Fredman. The researchers subsequently found that a molecule called CD47 on the necroptotic cells may act as a “don’t eat me” signal, slowing their engulfment by macrophages. Adding SPMs, however, boosts macrophages’ efferocytosis responses enough to overcome that signal.
Speaker Presentations
Further Readings
Ridker
Aday, A.W., Lawler, P.R., Cook, N.R., et al. (2018).
Circulation. 2018.
Cook, N.R., Mora, S., and Ridker, P.M.
Lipoprotein(a) and Cardiovascular Risk Prediction Among Women.
J. Am. Coll. Cardiol. 2018;72(3):287–296.
Mora, S., Wenger, N.K., Cook, N.R., et al.
JAMA Intern Med. 2018.
Conte
Almasri, J., Adusumalli, J., Asi, N., et al.
J. Vasc. Surg. 2018;68:624-633.
van Haelst, S.T.W., Teraa, M., Moll, F.L., et al.
J. Vasc. Surg. 2018;S0741-5214(18):30422-1.
McDermott, M.M., Spring, B., Berger, J.S., et al.
JAMA. 2018;319(16):1665-1676.
Fredman
Cai, B., Thorp, E.B., Doran, A.C., et al.
MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation.
Proc. Natl. Acad. Sci. U.S.A. 2016;113(23):6526–6531.
Doran, A.C., Ozcan, L., Cai, B., et al.
J. Clin. Invest. 2017;127(11):4075–4089.
Yurdagul, A., Doran, A.C., Cai, B., et al.
Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis.
Front Cardiovasc Med. 2018;4:86.
Session 2b: Medical Mystery Tour
Speakers
Nan Chiang, PhD
Brigham and Women’s Hospital, Harvard Medical School
Jesmond Dalli, PhD
William Harvey Research Institute, QMUL
Kevin Tracey, MD
The Feinstein Institute for Medical Research
The Role of n-3 Docosapentaneoic Acid-derived Pro-resolving Mediators in Systemic Protection
Jesmond Dalli, of the William Harvey Research Institute at the Queen Mary University of London, began the second part of the session with a presentation linking circadian rhythms and vascular inflammation. Disruption in circadian rhythms has been linked to metabolic disorders and cardiovascular disease progression, so Dalli wondered whether specialized pro-resolving mediators (SPMs) also showed circadian patterns.
Dalli’s team took blood samples from seven healthy volunteers throughout a 24-hour period, and found that the level of n-3 DPA, a precursor to SPMs, rose and fell in a consistent cycle. In contrast, patients with cardiovascular disease lose this circadian regulation, and have consistently low levels of SPMs. Additional experiments in mice confirmed the link. “This suggests that [loss] in circadian regulation of this pathway leads to a breakdown [in] early morning activation of platelets and monocytes,” said Dalli, “and that this then promotes cardiovascular disease.” He and his colleagues found that the cholesterol-lowering drugs atorvastatin and pravastatin increase the production of two SPMs, reinforcing other groups’ findings that these drugs combat cardiovascular disease through multiple mechanisms.
Pro-resolving Receptors: Mechanisms and Signaling
Nan Chiang from Harvard Medical School talked about the cellular receptors that respond to SPMs. One well-characterized SPM, resolvin D2, is a potent promoter of inflammation resolution; even extremely low doses of resolvin D2 can limit peritonitis and enhance survival in animal models of sepsis.
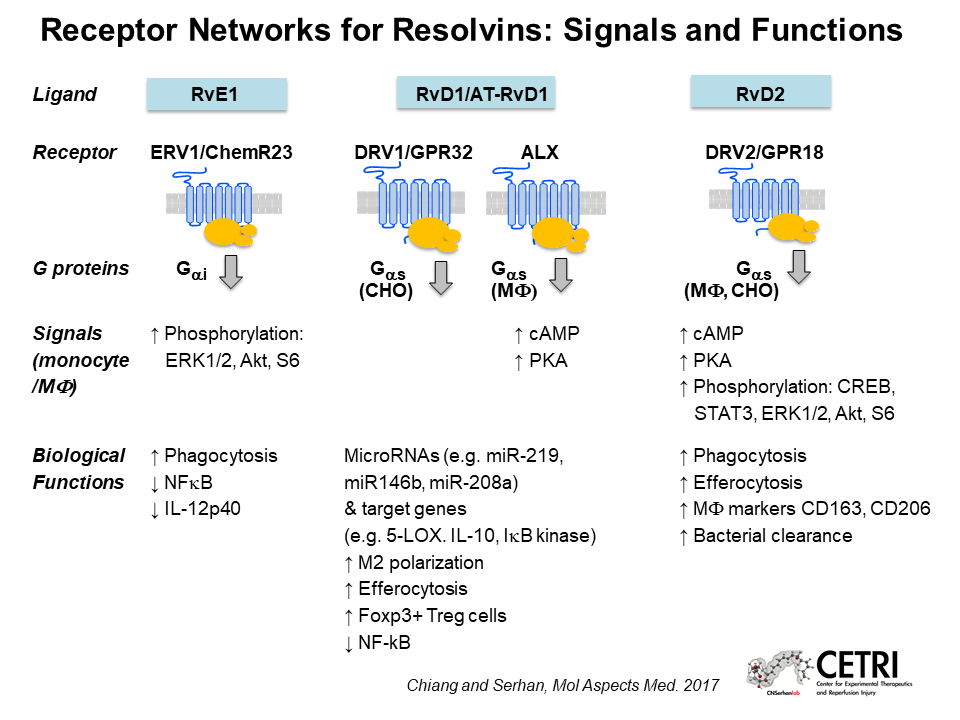
Chiang suspected that resolvin D2 might act through a G-protein coupled receptor, so she tested a panel of 77 of these receptors whose functions had not yet been identified. That screen revealed that the receptor GPR18 responds strongly to resolvin D2. Human macrophages overexpressing GPR18 respond more strongly to resolvin D2 than controls; and mice lacking GPR18 don’t benefit from resolvin D2 treatment during experimental E. coli infection or sepsis, confirming that GPR18 is a biologically relevant receptor for resolvin D2.
In addition to resolvins, Chiang also studies another family of SPMs, known as maresin conjugates in tissue regeneration (MCTRs). She discovered that MCTRs act through a receptor called CysLT1 to reduce vascular leakage after injury. Knowing the receptors for these SPMs should facilitate the design of drugs with similar effects.
Molecular Approaches to Bioelectronic Medicine
Kevin Tracey, from the Feinstein Institute for Medical Research, started the meeting’s second day with a keynote presentation on bioelectronic medicine. Instead of manipulating body chemistry with chemicals, bioelectronic treatments connect electronic devices to the nervous system to stimulate desired responses. “The idea of bioelectronic medicine is a mechanistic approach to using electrons to replace drugs,” Tracey explained.
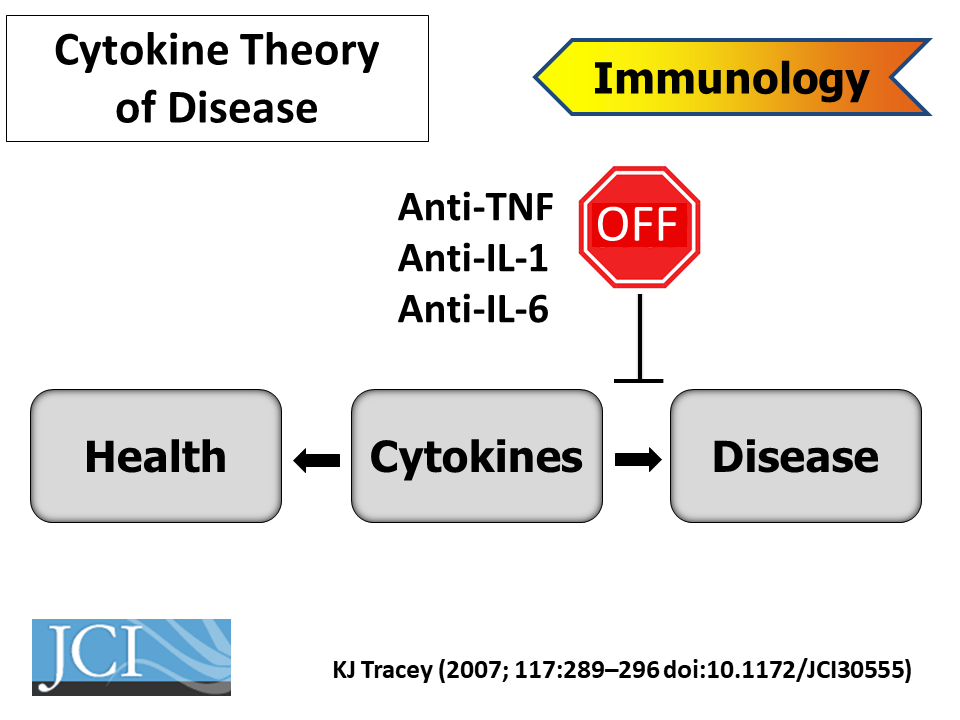
About thirty years ago, when Tracey was a practicing neurosurgeon, a young patient died in his arms from shock. That spurred him to search for new drugs that could block shock-causing cytokines. In one experiment, his lab injected a promising anti-inflammatory drug directly into the brains of mice with experimentally induced strokes. It blocked inflammatory cytokines in the brain, but also in other organs throughout the animals’ bodies. That made no sense, as the molecule shouldn’t have been able to escape the brain.
The researchers subsequently discovered that the vagus nerve can trigger an anti-inflammatory reflex throughout the body, connecting the nervous system directly to the immune system. Subsequent work by others revealed that the vagus nerve stimulates the production of pro-resolving mediators in multiple organs and tissues. Tracey’s team is now conducting clinical trials to treat Crohn’s disease with an implanted electronic device that stimulates the vagus nerve and triggers this response.
Speaker Presentations
Further Readings
Dalli
Arnardottir, H., Orr, S.K., Dalli, J., and Serhan, C.N.
Human milk proresolving mediators stimulate resolution of acute inflammation.
Mucosal Immunol. 2016;9(3):757–766.
Serhan, C.N., Chiang, N., Dalli, J., and Levy, B.D.
Lipid mediators in the resolution of inflammation.
Cold Spring Harb Perspect Biol. 2014;7(2):a016311.
Serhan, C.N., Chiang, N., and Dalli, J.
The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution.
Semin. Immunol. 2015;27(3):200–215.
Chiang
Chiang, N., Riley, I.R., Dalli, J., et al.
FASEB J. 2018;32(7):4043-4052.
Krishnamoorthy, S., Recchiuti, A., Chiang, N., et al.
Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs.
Am. J. Pathol. 2012;180(5):2018–2027.
de la Rosa, X., Norris, P.C., Chiang, N., et al.
Am. J. Pathol. 2018;188(4):950-966.
Tracey
Gunasekaran, M., Chatterjee, P.K., Shih, A., et al.
Immunization Elicits Antigen-Specific Antibody Sequestration in Dorsal Root Ganglia Sensory Neurons.
Front Immunol. 2018;9: 638.
Zanos, T.P., Silverman, H.A., Levy, T., et al. (2018).
Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity.
Proc. Natl. Acad. Sci. U.S.A. 2018; 115(21):E4843-E4852.
Session 3: Better Living through Chemistry
Speakers
Patricia Sime, MD
University of Rochester School of Medicine
Matthew Spite, PhD
Brigham and Women’s Hospital, Harvard Medical School
Temporal Biosynthesis of Pro-Resolving Lipid Mediators by Distinct Immune Cell Subsets during Skeletal Muscle Injury and Regeneration
Matthew Spite, of Brigham and Women’s Hospital and Harvard Medical School, returned the focus to chemistry, with a discussion of the specialized pro-resolving mediators (SPMs) that orchestrate healing from injuries. Using mass spectrometry, Spite identified all of the lipids produced in an injured mouse muscle as it recovers. The lipid profile changes over time and increases in the well-known resolvin subset of SPMs correlate with healing. That correlation persists whether the muscle damage comes from a toxin or exercise. “A lot of these pathways were consistent in two distinct models of injury,” said Spite. His team next characterized the macrophages present in the healing tissue, and found a distinct shift from pro-inflammatory to pro-resolving macrophage types, in sync with the shift in the lipid profile.
COPD: Inflammation, Infection and Resolution
Patricia Sime, from the University of Rochester School of Medicine, discussed the role of SPMs in chronic obstructive pulmonary disease (COPD). COPD, a chronic inflammatory condition, is now the fourth leading cause of death worldwide. It is caused primarily by smoking. “There’s actually no drug treatment for COPD,” said Sime, “so it’s a huge unmet need.”
To better understand COPD, Sime used mass spectrometry to profile the lipids in exhaled breath condensates from patients with the disease as well as healthy controls. That pointed toward the SPMs resolvin D1 and D2 as critical mediators of inflammation resolution in the lung. Treating a mouse model of chronic smoking with resolvins decreases inflammation in the animals’ lungs. Sime hopes to exploit these findings to develop new therapies for COPD, as well as prophylaxis against lung damage from all types of smoke inhalation.
Speaker Presentations
Further Readings
Spite
Hellmann, J., Sansbury, B.E., Wong, B., et al.
Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair.
J. Invest. Dermatol. 2018;(18)31728-7.
Winkler, J.W., Libreros, S., De La Rosa, et al.
J. Leukoc. Biol. 2018.
Wu, B., Werlin, E.C., Chen, M., et al.
Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rabbit vein graft model.
J. Vasc. Surg. 2018;(18)31349-1.
Sime
Bhat, T.A., Kalathil, S.G., Bogner, P.N., et al.
Secondhand Smoke Induces Inflammation and Impairs Immunity to Respiratory Infections.
J. Immunol. 2018;200(8):2927-2940.
Croasdell, A., Lacy, S.H., Thatcher, T.H., et al.
J. Immunol. 2016;196(6):2742-2752.
Lugade, A.A., Bogner, P.N., Thatcher, T.H., et al.
J. Immunol. 2014;192(11): 5226-5235.
Session 4: The Hunger Flames
Catherine Godson, PhD
University College Dublin
Mark Tepper, PhD
Corbus Pharmaceuticals
Thomas Van Dyke, DDS, PhD
Forsyth Center for Clinical and Translational Research
Lipoxins and Lipoxin Mimetics Attenuate Diabetic Complications
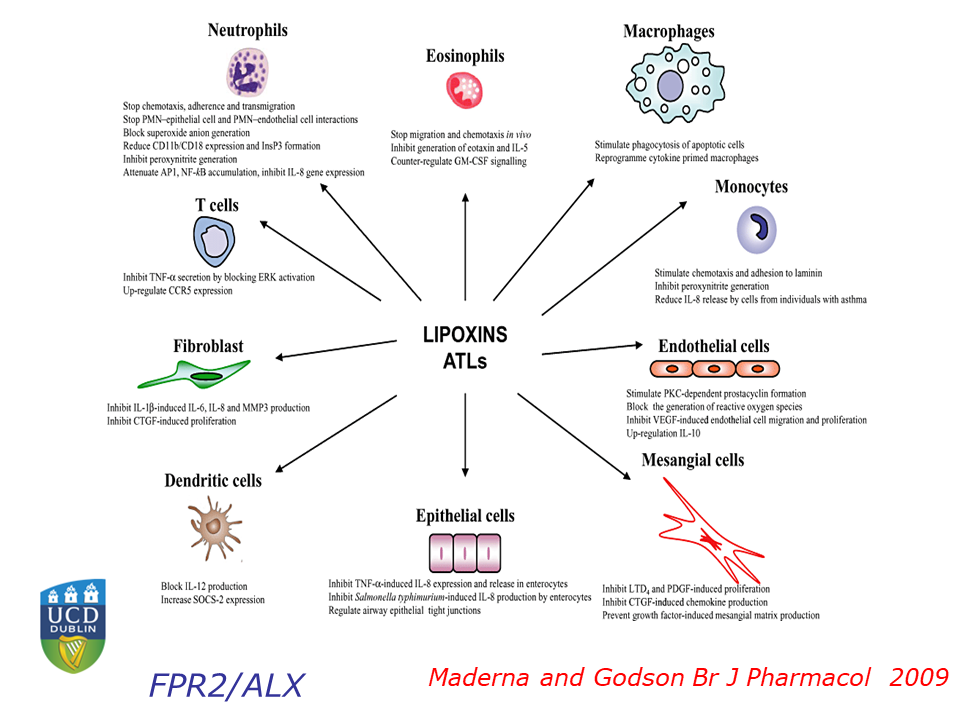
Catherine Godson of University College Dublin opened the meeting’s final session with a presentation on lipoxins in diabetes. As a result of the obesity pandemic, diabetes has become an immense global health problem. One of the most serious complications of diabetes is kidney disease. “Those with the most profound diabetic kidney disease… have almost 50% increased mortality,” said Godson, adding that “there are no effective therapeutics” for the condition.
In an effort to change that, Godson and her colleagues have examined the lipoxin A4 molecule, a specialized pro-resolving molecule (SPM) that attenuates inflammation in animal models. The researchers found that lipoxin A4 attenuates molecular signals that promote fibrosis, helps maintain epithelial tissue integrity, and reduces activation of the inflammatory cytokine TGF-beta. “All of these are important potential targets of lipoxin actions in the context of chronic kidney disease and diabetic kidney disease,” said Godson.
Next, Godson investigated lipoxin A4’s effects in animal models. Mice fed a high-fat diet become obese and develop kidney and liver disease similar to that seen in obese humans. Treating the animals with lipoxin A4 protects their livers and kidneys. Godson and her colleagues have also found that lipoxin A4 can reverse atherosclerosis in the overfed mice.
To translate those findings into practical medications, Godson is now working with synthetic chemists to develop drugs that can mimic the effects of lipoxin A4 in humans.The team has developed several promising leads, and is now testing them in various preclinical models.
Targeting the Endocannabinoid-Specialized Pro-resolution Mediator Pathway with Lenabasum to Treat Chronic Inflammatory/Fibrotic Diseases
Mark Tepper from Corbus Pharmaceuticals discussed lenabasum, the company’s synthetic, oral, small-molecule, selective cannabinoid receptor type 2 (CB2) drug. The endocannabinoid system activates the resolution of inflammation through the CB2 receptor. This GPCR is commonly found on activated immune cells during inflammation.
Based on promising preclinical results, Corbus tested lenabasum in the human clinical study (“blister model”) that has been designed to follow the progression of an inflammatory stimulus and subsequent resolution of inflammation. This study showed that lenabasum indeed stimulates inflammation resolution, reduces pro-inflammatory mediators, and promotes the clearance of bacterial endotoxins.
Corbus is now conducting more advanced clinical trials in patients with various chronic inflammatory conditions, including scleroderma, dermatomyositis, cystic fibrosis, and lupus. So far, the safety profile has been favorable with no serious adverse events attributed to lenabasum and no evidence of immunosuppression.
The Role of Resolution Phase Mediators in Oral Medicine
Thomas Van Dyke, from the Forsyth Center for Clinical and Translational Research, gave the final presentation: a look inside the mouth. Over 90% of American adults over age 30 suffer from gingivitis, and about 14% of them – or 22 million people – have severe periodontal disease. Conventional anti-inflammatory drugs can slow the progression of this disease, but can’t stop it.
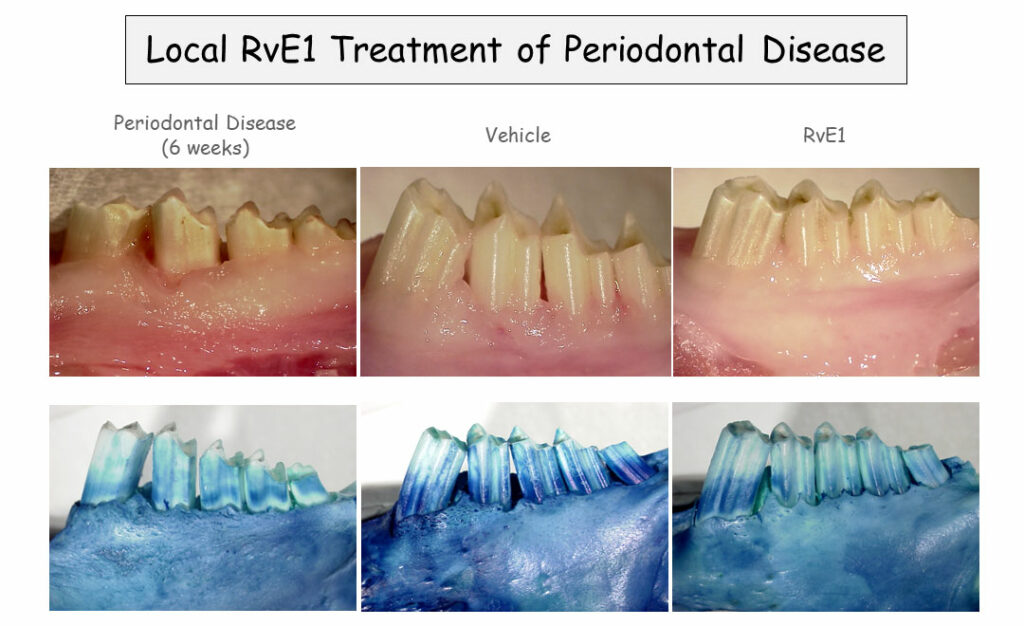
In a rabbit model of periodontitis, Van Dyke has found that rinsing the animals’ mouths with a SPM analog called benzo-lipoxin A4 reverses periodontitis and restores lost bone in the jaw. That’s far better than any previously studied treatment. “There is no [prior] example of pharmacologically induced bone regeneration in periodontal disease anywhere, this just doesn’t happen,” said Van Dyke. Additional studies in pigs yielded similar results, and Van Dyke and his colleagues are now conducting a clinical trial on benzo-lipoxin A4-containing mouthwash in patients with periodontitis.
Panel Discussion: Barriers to Translation in Inflammatory Disease
The meeting concluded with a wide-ranging panel discussion with extensive audience interaction, featuring speakers Godson, Sime, Conte, Tepper, and Van Dyke, and led by keynote presenter Serhan. Panelists covered issues that ranged from the need for better biomarkers to follow inflammation resolution in laboratory and clinical studies; to the difficulty of overcoming old paradigms of anti-inflammatory interventions in medicine; and the complex interplay between drug-based and “alternative medicine” strategies for promoting inflammation resolution.
Speaker Presentations
Further Readings
Godson
Brennan, E., Wang, B., McClelland, A., et al.
Diabetes. 2017; 66(8):2266–2277.
Brennan, E.P., Nolan, K.A., Börgeson, E., et al.
Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFβR1.
J. Am. Soc. Nephrol. 2013;24(4):627–637.
Brennan, E.P., Mohan, M., McClelland, A., et al.
Lipoxins Regulate the Early Growth Response-1 Network and Reverse Diabetic Kidney Disease.
J. Am. Soc. Nephrol. 2018;29(5):1437–1448.
Tepper
Burstein, S.H., and Tepper, M.A.
In vitro metabolism and metabolic effects of ajulemic acid, a synthetic cannabinoid agonist.
Pharmacol Res Perspect. 2013;1(2):e00017.
Motwani, M.P., Bennett, F., Norris, P.C., et al.
Clin. Pharmacol. Ther. 2017.
Van Dyke
Chapple, I.L.C., Mealey, B.L., Van Dyke, T.E., et al.
J. Periodontol. 2018;89 Suppl 1: S74–S84.
El Kholy, K., Freire, M., Chen, T., and Van Dyke, T.E.
Resolvin E1 Promotes Bone Preservation Under Inflammatory Conditions.
Front Immunol. 2018;9: 1300.
Viniegra, A., Goldberg, H., Çil, Ç., et al.
Resolving Macrophages Counter Osteolysis by Anabolic Actions on Bone Cells.
J. Dent. Res. 2018;97(10):1160-1169.
