Inside the Quest for a COVID-19 Vaccine
Reported by:
Mercedes Vega Villar
Presented by:
Microbiology and Infectious Diseases Discussion Group
The New York Academy of Sciences
Overview
When SARS-CoV-2—the respiratory virus that causes COVID-19—first emerged, most people did not anticipate that it would result in a global public health disaster. COVID-19 rapidly spread from person to person across all borders, bringing hospitals to the brink of collapse, causing a devastating loss of life, and shutting down global economies. Scientific researchers, biotechnology companies, and government agencies quickly mobilized to develop vaccines—which prevent disease in inoculated individuals and, in some cases, also block a pathogen’s transmission from person to person—against SARS-CoV-2. The unprecedented speed of SARS-CoV-2 vaccine development reflects decades of previous research on similar coronaviruses and faster manufacturing techniques. Just over a year into the pandemic, there are already candidate vaccines for SARS-CoV-2, several of which are being rolled out worldwide. Many other vaccine candidates are currently being investigated and will hopefully become part of the toolkit in the fight against COVID-19.
On February 2-3, 2021, the New York Academy of Sciences hosted a historic symposium that brought together top virologists and vaccinologists, public health officials, and industry leaders. They reflected on the factors that contributed to the record-breaking speed of COVID-19 vaccine development, gave updates on vaccine candidates, reviewed strategies to stay ahead of future outbreaks, and discussed the many unanswered questions and challenges that lie ahead.
Symposium Highlights:
- Decades of previous research in virology and vaccinology sped up COVID-19 vaccine development. Productive public-private coordination was also critical. >
- Various vaccines using a range of technology platforms are currently being developed. >
- Several COVID-19 vaccines have proven to be safe and immunogenic in Phase 1 and 2 clinical trials. Some of them have met safety and efficacy standards in Phase 3 trials and are already in the market in several countries. >
- The emergence of new variants of SARS-CoV-2 is a source of concern for vaccine experts, but they remain optimistic. More data is still needed, but the vaccines that are already being rolled out or close to it seem to confer some degree of protection against the known variants. >
- Many questions remain unclear, such as the duration of the protective effects of vaccines or the effects of COVID-19 vaccines in children. >
- Investing in research and prevention strategies to bridge the pandemic preparedness gap is essential in the effort to stay ahead of future outbreaks. >
Keynote Speakers
Anthony S. Fauci, MD
National Institute of Allergy and Infectious Diseases (NIAID), NIH
Moncef Slaoui, PhD
Operation Warp Speed
Speakers
Sara Gilbert, PhD
University of Oxford
Gregory Glenn, MD
Novavax
Kathrin Jansen, PhD
Pfizer
Kevin Olival, PhD
EcoHealth Alliance
Stanley Plotkin, MD
University of Pennsylvania
Melanie Saville, MD
CEPI
Hanneke Schuitemaker, PhD
Janssen Vaccines and Prevention B.V.
Xuefeng Yu, PhD
CanSino Biologics
Tal Zaks, MD, PhD
Moderna
Sponsors
Gold Sponsors



Bronze Sponsor
Promotional Partners
Keynote Address – Slaoui
Speakers
Moncef Slaoui
Operation Warp Speed
Operation Warp Speed (OWS) and the Quest for a COVID-19 Vaccine
The Operation Warp Speed (OWS) program, initiated by the federal government, was designed to accelerate the development and distribution of COVID-19 vaccines. Moncef Slaoui, former chief scientific officer of OWS offered a broad overview of the program, the status of candidate vaccines, and key lessons from the vaccine development process.
He declared the success of the ambitious mission, which allowed for the delivery of tens of millions of vaccines in the US by February 2021. “It is remarkable that we are at that level twelve months and a few days after the virus was described,” said Slaoui. He credited this success in part to the collaborative efforts of researchers around the world, as well as the cooperation between the various government agencies and private sector partners. “This level of coordination under one leadership was unprecedented,” remarked Slaoui.
The program’s “portfolio approach,” which supported simultaneous research for 6-8 candidate vaccines, was also critical to its effectiveness. This allowed for a high level of attrition and increased capacity of manufacturing doses. Under Slaoui’s leadership, OWS also maximized speed by enabling the development, clinical trial, and manufacturing processes to proceed in parallel. Typically, manufacturing plans are not decided until after conducting the clinical trials. This strategy proved to be worth the risk when the first Phase 3 trial results from the mRNA vaccines revealed an efficacy of 95%. Likewise, OWS facilitated rapid clinical testing with little lag time between the different trial stages, and it helped private companies develop the needed manufacturing capabilities.
Slaoui, who emphasized the need for better pandemic preparedness, pointed to the spread of misinformation on vaccines and public mistrust as an “extremely disappointing dimension” that can be blamed on the politicization of the pandemic. Although the veteran vaccinologist noted OWS’s inability to effectively manage the public’s expectations and anticipate problems with distribution and delivery of the vaccine at the state level, he believes the development of multiple candidate vaccines is a monumental success.
Further Readings
Slaoui
Slaoui M, Hepburn M.
Developing Safe and Effective Covid Vaccines – Operation Warp Speed’s Strategy and Approach
N Engl J Med. 29 Oct 2020;393(18):1701-1703.
Slaoui M, Greene SE, Woodcock J.
Bridging the Gap at Warp Speed – Delivering Options for Preventing and Treating Covid-19
N Engl J Med. vol. 383,20 (2020): 1899-1901.
Keynote Address – Fauci
Speakers
Anthony S. Fauci, MD
National Institute of Allergy and Infectious Diseases (NIAID), NIH
This Year in Review: A Vaccinologist’s Perspective
Anthony Fauci, director of the National Institutes of Allergy and Infectious Diseases (NIAID), explained how it was possible to develop COVID-19 vaccines in months, when the time to develop other vaccines “had historically been measured in years.” The groundwork laid by decades of vaccine research deserves much of the credit. He traced the COVID-19 vaccine origin to 1996, when a conversation about HIV vaccine research he had with President Clinton led to the start of the NIAID Vaccine Research Center. The center—whose mission eventually grew to include other pathogens—started as an interdisciplinary effort for scientists to collaborate on research and clinical trials for an HIV vaccine.
Fauci discussed research by his colleague Peter Kwong, a structural biologist who, in 2014, mapped the envelope protein that could serve as a suitable target for a HIV-1 structure-based vaccine design. Kwong’s techniques were adapted for the development of a vaccine against other respiratory viruses. Thanks to his work, when SARS-CoV-2 appeared, researchers were able to quickly elucidate that a specially modified version of the coronavirus’ spike protein was the best antigen candidate for a vaccine.
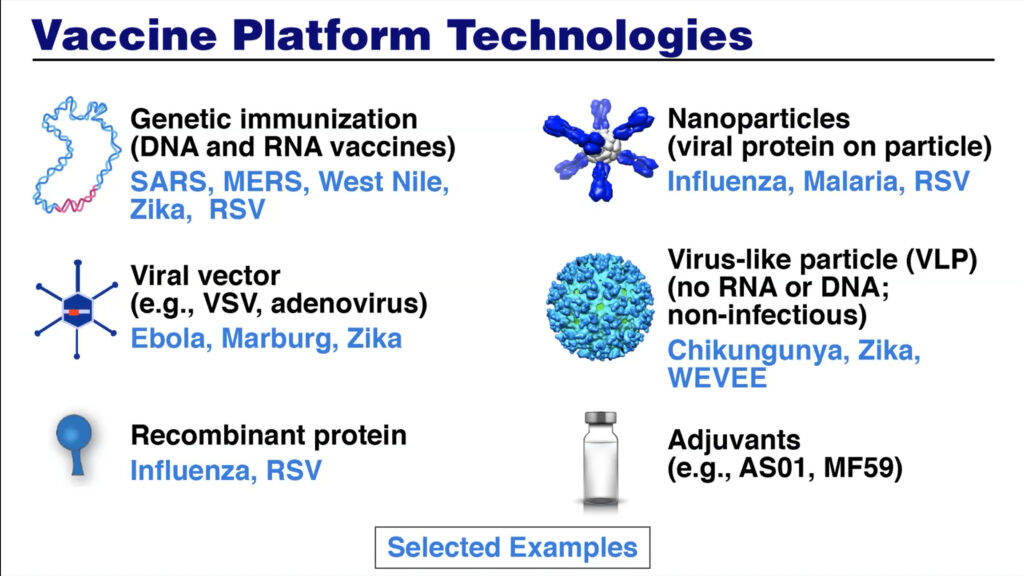
Additional platforms—including mRNA, recombinant proteins, genetically engineered viral vectors— currently used for COVID-19 vaccines were previously investigated and developed for other vaccines at the NIAID Vaccine Research Center. This scientific foundation, combined with the coordination of resources and agencies and a “harmonization of goals,” allowed for rapid vaccine development. To speed up clinical trials, “the extraordinary investments that were made decades ago in putting together the HIV clinical trial network were immediately adapted,” said Fauci.
Although Fauci recognizes the challenges of distribution, he remains optimistic. “The hope is that, when we get to the end of the spring and into the summer,” said Fauci, “we can have the overwhelming majority of people vaccinated.” He estimated that “75-80% need to be vaccinated and/or protected by previous infection” for herd immunity to be achieved. He also expressed concerns about the significant proportion of Americans that are hesitant about getting the vaccine. “We need to respect that, but we need to try and convince them of the importance, for their own safety and the safety of their family and the American public, to get vaccinated,” he added. Fauci is confident that the techniques developed will allow for easy adaptions of the current vaccines to protect against SARS-CoV-2 mutations. The development of universal coronavirus vaccines, which is necessary to stay ahead of new coronaviruses, will hopefully be the next step.
Further Readings
Fauci
Pancera M, Zhou T, Druz A, et al.
Structure and immune recognition of trimeric pre-fusion HIV-1 Env
Nature. 2014;514(7523):455-461.
Wrapp D, Wang N, Corbett KS, et al.
Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation
Science. 2020;367(6483):1260-1263.
Corey L, Mascola JR, Fauci AS, Collins FS.
A strategic approach to COVID-19 vaccine R&D
Science. 29 2020 May; 368(6494):948-950.
Giurgea LT, Han A, Memoli MJ.
Universal coronavirus vaccines: the time to start is now
NPJ vaccines. 28 May 2020;5(43).
Efficacy Studies, Part One
Speakers
Gregory Glenn, MD
Novavax
Kathrin Jansen, PhD
Pfizer
Tal Zaks, MD, PhD
Moderna
Efficacy Data Updates from Moderna’s mRNA Vaccine Candidate
Tal Zaks, chief medical officer of Moderna, gave an overview of the company’s efforts to create and distribute a COVID-19 vaccine and ensure protection against new virus variants.
Zaks pointed to three factors he believes led to the creation of a vaccine in only 11 months. First, the science already existed. The mRNA platform’s central concept, which is that “you can teach a cell how to make a protein by providing it with mRNA,” was proven and shown to create neutralizing antibodies against SARS-CoV-2. Secondly, a sense of urgency due to the pandemic’s severity allowed the clinical trials to proceed quickly but, Zaks assured, “without cutting corners.” It is an unfortunate “paradox of vaccine development” Zaks explained, that the more cases occur, “the faster you will know if a vaccine works.” Finally, he credited the speed of development to Moderna’s government stakeholders. “The unsung heroes are the FDA,” he said.
Zaks then highlighted the Phase 3 clinical trial results. The trial, which was representative of minorities and included mostly frontline workers, showed 94.1% efficacy of the vaccine. He described the adverse vaccine effects as non-severe and expected. Anaphylaxis, a life-threatening allergic reaction to injectable drugs, is of concern with all vaccines. The reaction occurs at the rate of 2.5 per one million doses of the Moderna COVID-19 vaccine administered.
Zaks also discussed new Covid-19 variants. Of most concern are viruses with mutations on the receptor binding domain or the N-terminal domain, which may “improve the virus ability to escape the immune response.” Researchers saw a drop in robustness of the vaccine in the B.1.351 variant, but the vaccine remained effective. Moderna will continue to monitor mutations over time while they research booster shots to combat new variants.
Efficacy Data Updates from the Pfizer-BioNTech mRNA Vaccine Candidate
According to Kathrin Jansen, senior vice president of vaccine research and development at Pfizer, a vaccine that relies on an mRNA platform has many advantages. For example, mRNA vaccines do not use viral foreign proteins, making them safe and easy to produce at scale. Also, they generate a broad immune response, which is helpful because our knowledge of what immune responses best correlate with protection is still limited. Jansen presented data indicating that the breakthrough Pfizer-BioNTech mRNA vaccine is extremely safe and 95% effective, but she also highlighted the many challenges that lie ahead.
For instance, while the clinical trials conducted in Germany and the US captured a diverse sample from a range of ages and ethnicities, critical segments of the population were excluded due to age or clinical conditions. Clinical trials with children 12-15 are currently underway, but trials with younger children will have to wait. Pfizer-BioNTech’s vaccine needs to be kept between -80°C and -60°C, complicating storage and distribution. Jansen noted they are “making progress in a vaccine formulation that won’t require such cold temperatures.”
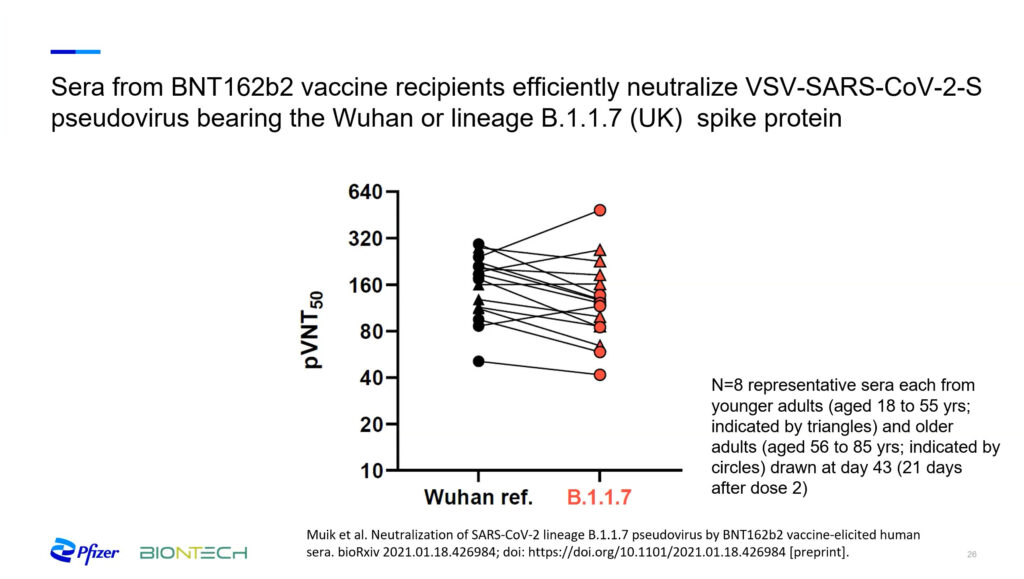
Highly transmissible variants that have emerged in the United Kingdom and South Africa pose what is perhaps the biggest challenge. These variants include mutations in the spike protein that Pfizer-BioNTech’s vaccine uses as a target. One of the approaches they use to research efficacy against the new variants involves creating synthetic viruses that express the mutations of interest. Then, they examine the neutralizing potential of blood sera extracted from vaccinated participants. Jansen said that data from these studies suggests that “this vaccine will continue to perform well against at least the variants that have appeared here.” However, she cautioned that this data “needs backing up by vaccine efficacy surveillance as well as animal models.”
Further Readings
Jansen
Walsh EE, Frenck RW Jr, Falsey AR, et al.
Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates
N Engl J Med. 17 Dec 2020;383(25):2439-2450.
Polack FP, Thomas SJ, Kitchin N, et al.
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
N Engl J Med. 31 Dec 2020;383(27):2603-2615.
Muik A, Wallisch A, Sänger B, et al.
Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera
Science. 29 Jan 2021.
Xie, Xuping et al.
Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera
bioRxiv. 7 Jan. 2021, Preprint.
Efficacy Data Updates from Novavax’s Protein-based Vaccine Candidate
Gregory Glenn gave an update on the progress of Novavax’s protein-based COVID-19 vaccine, which was not available to the public at the time of his presentation. Novavax’s recombinant nanoparticle technology produces a full-length prefusion spike protein. The protein is combined with a saponin-based Matrix-M™ adjuvant and encoded with the Sars Cov-2 spike, and produced in insect cells. Similar techniques have proven successful in Novavax influenza vaccines. Importantly, the vaccine can remain stable in a refrigerator for up to three months, lowering distribution and storage costs.
Glenn, the president of research and development at Novavax, explained that in the pre-clinical package, researchers showed protection in the lower and upper airways of Rhesus Monkeys and produced an antibody response in a trial with 131 clinically ill convalescent subjects. At the time of the presentation, Novavax was conducting its Phase 3 US/Mexico trial and did not have results. However, Glenn was able to report the results of trials in the UK and South Africa. In the UK, researchers found that the vaccine was effective at 94% for the ancestral Covid-19 strain, but decreased to 86% for the UK strain. In South Africa, where the new strain became dominant during the trial, the efficacy decreased but remained around 60%.
Based on these results, Novavax has started developing vaccines for the new variants. Glenn predicts that booster and bivalent vaccines “may become part of the annual influenza immunization regime.” The vaccines are even more important and urgent, Glenn argued, because their South African data showed that “herd immunity from previous infection is not working to protect against the new variant strain.” Glenn expressed optimism about their ability to scale up production, saying that “over the past year, we went from nothing to having eight manufacturing sites in seven countries.”
Further Readings
Glenn
Bangaru S, Ozorowski G, Turner HL, et al.
Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate
Science. 27 Nov 2020;370(6520): 1089-1094.
Keech C, Albert G, Cho I, et al.
Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine
N Engl J Med. 2020;383(24):2320-2332.
Efficacy Studies, Part Two
Speakers
Sara Gilbert, PhD
University of Oxford
Hanneke Schuitemaker, PhD
Janssen Vaccines and Prevention B.V.
Xuefeng Yu, PhD
CanSino Biologics
Update on ChAdOx1 nCoV-19/AZD1222
The Oxford-AstraZeneca adenovirus vaccine has an important advantage that distinguishes it from other vaccines currently on the market: it can be stored in a regular refrigerator for up to six months. Sarah Gilbert, professor at the University of Oxford and head of the team that developed the vaccine, emphasized the vaccine’s affordability, explaining that her team envisioned it as “a vaccine for the world.” The Oxford-AstraZeneca vaccine is being tested in clinical trials in many countries. “It was important to us to get the information on how the vaccine behaves in different populations across the world,” she said.
An early report indicated that the two-dose Oxford-AstraZeneca vaccine was about 70% efficacious at preventing COVID-19. Closer examination of the data, however, led Gilbert and her team to realize that the timing of the second dose was critical: efficacy was only 50%-60% when doses were administered less than two months apart, but waiting three months boosted efficacy levels up to 82.4%. Waiting three months to give the second dose is now the policy in the UK, the first country to grant emergency authorization for the vaccine. Gilbert and her team also found that the first dose alone is highly efficacious (76%) at protecting against COVID-19, but only for the first three months. This is enough time to reduce the risk of people contracting the disease while they wait for the booster dose.
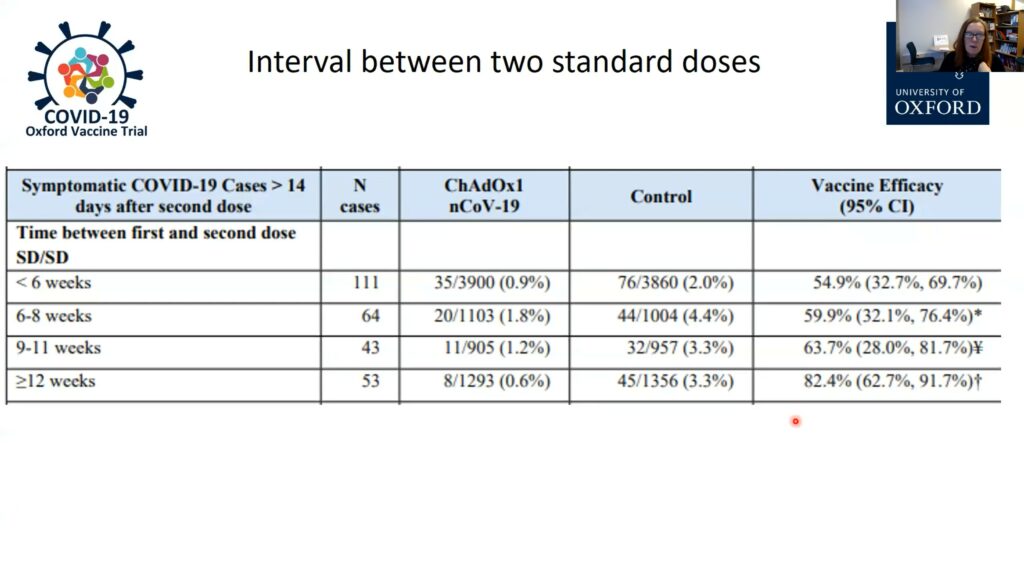
Gilbert also suggested the Oxford-AstraZeneca vaccine may be able to help curb the transmission of the virus. During clinical trials in the UK, nasal swabs of all participants were collected weekly. The scientists found 67% fewer positive samples in the vaccinated group compared to the placebo group, and that this included asymptomatic cases. The Oxford-AstraZeneca vaccine has obtained emergency approval in 23 countries so far, and the plan is to manufacture 3 billion doses by the end of 2021.
Further Readings
Efficacy Data Updates from CanSino Biologics’ Viral-Vector Vaccine Candidate
Xuefeng Yu, chairman of CanSino Biologics, provided an overview of their COVID-19 vaccine and described the China-based company’s efficacy and safety research. The CanSino Biologics’ Ad5-nCov vaccine is built on an adenovirus-based viral vector platform, a mechanism similar to the one used in the Oxford-AstraZeneca and Johnson & Johnson vaccines. Yu announced that, pending final analysis of its Phase 3 clinical trial, the company plans to file for emergency authorization in several countries soon.
The Ad5-nCov vaccine was approved for limited use by the Chinese military in June 2020. Phase 1 and 2 clinical trials conducted in Wuhan indicated the vaccine is safe and induced significant immune responses after a single dose. Over 150,000 members of the Chinese military have received a dose of the vaccine. “We haven’t had any severe adverse events in that population,” said Yu before explaining that efficacy is difficult to assess in China because “there are really no cases right now.”
CanSino Biologic’s Phase 3 clinical trial for the vaccine has been taking place in five countries since September, with Pakistan and Mexico providing the majority of the 40,000 participants. Yu explained the clinical trial results are not available to the company, which is still blinded to the treatment groups. However, recent data analyses by an independent committee has declared the vaccine meets primary safety and efficacy criteria.
The Phase 3 clinical trial for the Ad5-nCov vaccine differs from others in two critical ways. First, the vaccine’s long-term efficacy will be tested by tracking a subset of participants for one year. They are also testing a two-dose trial that includes children as young as six years old, but that data is not yet available.
Further Readings
Yu
Zhu F-C, Li Y-H, G X-H, et al.
Lancet. 13 Jun 2020;395(10240):1845-1854.
Zhu F-C, et al.
Lancet. 15 Aug 2020; 396(10249): 479-488.
Janssen’s Effort in the Development of an Ad26 Based COVID-19 Vaccine
The COVID-19 vaccine developed by Janssen, a pharmaceutical division of Johnson & Johnson, has just been authorized for emergency use in the US. Hanneke Shuitemaker, head of Viral Vaccine Discovery at Janssen Vaccines & Prevention B.V., explained that their Ad26.COV2.S vaccine relies on a proprietary adenovirus technology that the European Commission first approved in July 2020, in the context of an Ebola vaccine.
Phase 1 and 2a clinical trials recruited adults of all ages, including 375 participants over 65 years old. These trials revealed that the Ad26.COV2.S vaccine is safe, and most side effects were mild or moderate. The participants who were more likely to experience adverse events were younger participants and those who received the higher dose of the vaccine. Notably, both dose levels demonstrated similar immunogenicity in all age groups. Hence, Shuitemaker and her team decided to test the lower dose of their Ad26.COV2.S vaccine in Phase 3 clinical trials.
Last September, Janssen launched a Phase 3 clinical trial called ENSEMBLE, which tested the efficacy of a single dose regimen across the US, South Africa, and Latin American countries. The ENSEMBLE trial revealed that a single-dose of the Ad26.COV2.S vaccine had a 66% overall efficacy at preventing moderate to severe COVID-19. The vaccine was highly efficacious against severe disease (85%), and it provided 100% protection against COVID-19-related hospitalization and death. In the South African trial, where 97% of the infections from which SARS-CoV-2 sequence data was available, involved the new B.1.351 variant, the vaccine showed the same efficacy levels against severe disease and hospitalizations.
Although Janssen’s vaccine is not quite as efficacious against moderate COVID-19 as other vaccines already on the market, it is highly efficacious against severe COVID-19, hospitalization, and death. In addition, the one-dose vaccine does not need to be stored in ultracold temperatures and confers protection against new variants. “Overall, we are very happy with this outcome,” Shuitemaker said. “At the beginning of this journey, we had established that a single-dose vaccine with 70% efficacy would be a tremendous tool in the fight against this pandemic,” she added. A second Phase 3 clinical trial (ENSEMBLE 2), which tests the efficacy of a two-dose vaccine regimen, is currently underway.
Further Readings
Schuitemaker
Bos R, Rutten L, van der Lubbe JEM, et al.
NPJ Vaccines. 28 Sep 2020; 5:91.
Mercado NB, Zahn R, Wegmann F, et al.
Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques
Nature. 2020; 586(7830):583-588.
Sadoff J, Le Gars M, Shukarev G, et al.
Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine
N Engl J Med. 13 Jan2021 NEJMoa2034201.
Outbreak Predictions and Future Considerations
Speakers
Kevin Olival, PhD
EcoHealth Alliance
Stanley Plotkin, MD
University of Pennsylvania
Melanie Saville, MD
CEPI
Challenges to Prediction and Prevention of the Next Pandemic Zoonosis
According to Kevin Olival, vice president of research at EcoHealth Alliance, the threat of emerging infectious diseases has been rising for the last 70 years. Most of these infectious diseases are viral and linked to interactions between humans and wildlife. He explained that wild animals may host a diversity of viruses and that some of these viruses have the potential to infect human cells, inducing what is known as zoonotic diseases. Identifying the viruses that are more likely to jump from other species to humans and interrupting interactions between humans and the animals that carry those viruses is a challenging yet promising strategy to prevent future pandemics. In fact, two years before the COVID-19 pandemic emerged, Olival and his team published a study warning about villagers in the Yunnan province (China) being highly exposed to bats that carried SARS-related coronaviruses.
Not surprisingly, predicting where a novel infectious disease will emerge is very difficult. For instance, cataloguing all the viruses that can potentially infect each animal species involves intensive fieldwork. “Often people make the analogy with weather prediction, which was very coarse 50 years ago and we couldn’t see hurricanes coming weeks in advance,” Olival said of this nascent and complex science.
Given the multi-disciplinary and global nature of this kind of research, a centralized data platform to allow researchers to share and combine their findings will be critical. “These disparate data sets need to be put together,” said Olival.
Finally, he advocated for the need to shift policy towards pandemic prevention. It’s critical to get “policymakers to realize that there are other ways to deal with emerging infectious diseases than waiting for them to emerge and then responding,” said Olival. Once a high-risk hotspot has been identified, low-tech behavioral interventions to prevent human-animal contact may be all that is need to prevent a potentially devastating global pandemic.
Further Readings
Olival
Wang N, Li S-Y, Yang X-L, et al.
Serological Evidence of Bat SARS-Related Coronavirus Infection in Humans, China
Virologica Sinica. 2018 Feb;33(1):104-107.
Allen T, Murray KA, Zambrana-Torrelio C, et al.
Global hotspots and correlates of emerging zoonotic diseases
Nature communications. 24 Oct 2017;8(1):1124.
Latinne A, Hu B, Olival KJ, et al.
Origin and cross-species transmission of bat coronaviruses in China
Nature communications. 25 Aug 2020;11(1):4235.
United Nations Environment Programme and International Livestock Research Institute.
Preventing the Next Pandemic: Zoonotic diseases and how to break the chain of transmission
6 Jul 2020.
Lessons Learned from COVID-19 Vaccine Development for Future Pandemic Preparedness
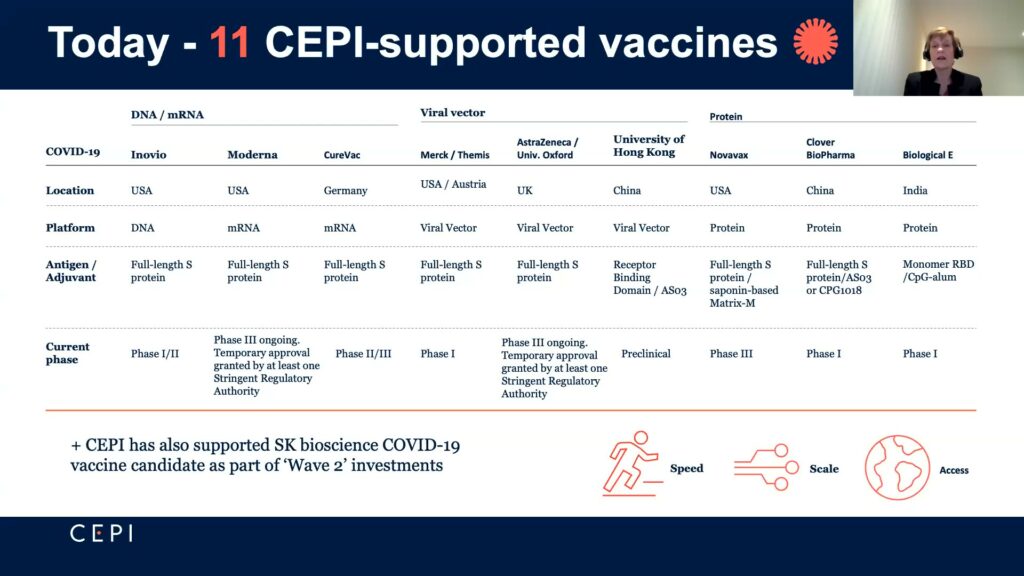
Melanie Saville, director of vaccine research and development at the Coalition for Epidemic Preparedness Innovations (CEPI), outlined the organization’s journey through COVID-19 vaccine development and lessons learned. Created in 2017 in response to the Ebola outbreak in West Africa, CEPI seeks to “accelerate vaccines for emerging infectious diseases and ensure equitable access to the vaccines,” said Saville. Prior to COVID-19, CEPI focused mainly on MERS and rapid response platforms like mRNA. This put CEPI on good footing when they shifted focus to COVID-19 at the start of January 2020.
By April of 2020, CEPI had raised over $1.5 billion in funding and entered partnerships with nine entities using varied strategies to develop COVID-19 vaccines. “Speed, scale and access,” the career virologist said, were the main criteria in determining investments. For speed, they carefully chose their partners and made early investments to ensure manufacturing capabilities to meet their accessibility goals of 2 billion vaccine doses worldwide by the end of 2021. That they invested in a portfolio of vaccines meant that if a vaccine failed, facilities could then be used for another vaccine. This manufacturing investment also helped with scalability, which is a problem particularly for smaller companies that have to resolve supply chain issues with sufficient materials and facilities.
CEPI joined the ACT Accelerator, established by the World Health Organization, to speed-up development of vaccines, diagnostics, and therapeutics and launched their taskforce, “Agility,” to better track variants. Saville sees these coalitions and organizations as a model and foundation for future pandemic responses. Overall, she’s optimistic. The pandemic has created a global desire for countries to invest and work together. “We have seen a revolution in vaccinology,” said Saville.
Further Readings
Saville
Lurie N, Saville M, Hatchett R, Halton J.
Developing Covid-19 Vaccines at Pandemic Speed
N Engl J Med. 2020;382(21): 1969-1973.
Thanh Le T, Andreadakis Z, Kumar A, et al.
The COVID-19 vaccine development landscape
Nat Rev Drug Discov. 2020 May;19(5): 305-306.
The Plague Year of 2020 and Its Effect on Vaccinology
In the final talk of the symposium, vaccinologist Stanley Plotkin reflected on how SARS-CoV-2 has impacted vaccinology. He praised the “all hands on deck” approach that we witnessed in 2020, with experts around the world getting involved and collaborating to develop multiple highly effective vaccines. Plotkin was also optimistic about the effect that the pandemic has had on vaccine acceptance. “Now, most people in all countries are pleading for vaccines, and to me that is a positive thing,” he said.
He also highlighted the importance of virology and other basic sciences. He explained that a handful of coronavirus researchers did the work that became the cornerstone of COVID-19 vaccines. According to Plotkin, “we need to support all those basic sciences, so that when we need something practical, we have the information we need to start working on a solution.”
Plotkin also listed a series of unknowns that researchers will need to figure out going forward. For example, the issue of mucosal responses to the vaccine. SARS-CoV-2 is a mucosal pathogen that takes hold in the nasal pharynx before spreading to the lungs and other organs. It is still unclear to what extent the current vaccines prevent mucosal replication. “Understanding how well they [prevent mucosal replication] has terribly important epidemiological implications regarding herd immunity and the spread of the disease,” he said.
Due to the tendency of SARS-CoV-2 to mutate, Plotkin said we have to face the possibility of a yearly vaccination. He advocated for the creation of regional labs that can monitor and quickly report on mutations across the world, something that is done with influenza. He also emphasized that we need to learn more about veterinary viruses, as they “have caused problems, are causing problems, and will cause problems.”
Further Readings
Plotkin
WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation, et al.
Placebo-Controlled Trials of Covid-19 Vaccines – Why We Still Need Them
N Engl J Med. 2021 Jan 14;384(2):e2.
Plotkin SA.
Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2
J Pediatric Infect Dis Soc. 10 Nov 2020;9(5): 517-518.
Plotkin SA, Halsey N.
Accelerate COVID-19 Vaccine Rollout by Delaying the Second Dose of mRNA Vaccines
Clin Infect Dis. 27 Jan 2021;ciab068.
Plotkin S.
Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):12283-7. Epub 2014 Aug 18.